x

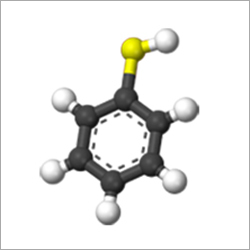
Thiophenol
1 INR/Kilograms
Product Details:
- Grade Industrial Grade
- Poisonous YES
- Physical Form Liquid
- Ph Level Not applicable (neutral compound)
- HS Code 29309020
- Melting Point -16 C
- Appearance Clear, colorless to pale yellow liquid
- Click to View more
X
Thiophenol Price And Quantity
- 1 INR/Kilograms
- 1 Kilograms
Thiophenol Product Specifications
- Thiophenol (Benzene Thiol)
- 1.5921.595
- 110.18 g/mol
- Store in tightly closed containers, away from heat and sources of ignition
- >=99%
- Strong, unpleasant, resembling rotten eggs
- Not for human consumption
- 203-635-3
- 1.07 Gram per cubic centimeter(g/cm3)
- Liquid form
- C6H6S
- C6H5SH
- Chemical Compound
- Insoluble in water; soluble in organic solvents
- Used as an intermediate in organic synthesis and as a reagent
- 2 years under recommended storage
- 108-98-5
- Clear, colorless to pale yellow liquid
- Pharmaceuticals, agrochemicals, dyestuffs, chemical synthesis
- Highly flammable; strong unpleasant odor; forms toxic fumes when heated
- 29309020
- Not applicable (neutral compound)
- -16 C
- Liquid
- Industrial Grade
- YES
Thiophenol Trade Information
- 25 Kilograms Per Week
- 3-4 Week
Product Description
Thiophenol
| Thiophenol | |
|---|---|
| Analysis Item | Specification |
| Purity | 99.0% Min |
| Appearance | Colorless to Slightly Yellowish |
| Diphenyl Disulfide | 1.00% Max |
| Chlorobenzene | 1.00% Max |
Applications of Thiophenol
Thiophenol finds appliction in Pharmaceutical, Dyes & Agrochemical synthesis, It also finds application Veterinary Drugs.
Key Properties and Composition
Thiophenol exhibits a boiling point of 169 C, a flash point of 57 C, and is insoluble in water but highly soluble in organic solvents. Its strong odor and toxic nature require cautious handling. The chemicals molecular formula is C6H6S, with a molecular weight of 110.18 g/mol, making it suitable for various industrial applications.
Safe Handling and Storage
Always store thiophenol in tightly closed containers away from direct heat or ignition sources. Utilize appropriate protective gloves and eye/face protection when handling. Its physical and chemical stability ensures a shelf life of up to two years if storage recommendations are followed precisely.
Industrial Uses and Applications
Widely used as an intermediate in organic synthesis, thiophenol plays a crucial role in manufacturing pharmaceuticals, agrochemicals, and dyestuffs. Its efficacy as a reagent stems from its high purity (99%), making it a reliable choice for chemical syntheses across various industries in India and beyond.
FAQs of Thiophenol:
Q: How should thiophenol be safely handled and stored?
A: Thiophenol should be handled using protective gloves and eye/face protection due to its high toxicity and flammability. Store the liquid in tightly closed containers away from heat, sparks, open flames, or hot surfaces to maintain stability and safety.Q: What is thiophenol commonly used for in the industry?
A: Thiophenol is mainly used as an intermediate in organic synthesis for producing pharmaceuticals, agrochemicals, and dyestuffs. Its strong reactivity and purity make it valuable for a variety of chemical manufacturing processes.Q: When is it necessary to take extra precaution with thiophenol?
A: Extra precaution should be taken whenever handling, transferring, or storing thiophenol due to its poisonous, highly flammable nature and strong, unpleasant odor. Exposure to heat, open flames, or inadequate ventilation should also be strictly avoided.Q: Where does thiophenol find its main applications?
A: Thiophenol is primarily employed in laboratories and industrial environments in India and globally, particularly in the synthesis of complex chemical compounds for pharmaceuticals, dye, and crop protection products.Q: What processes is thiophenol involved in during chemical synthesis?
A: Thiophenol acts as a reagent or intermediate in numerous organic synthesis reactions, particularly for manufacturing sulfonated compounds, pharmaceuticals, and agricultural chemicals due to its reactive thiol group.Q: What benefits does thiophenol offer in manufacturing?
A: Thiophenols high purity and reactivity enable efficient synthesis of target molecules, reducing impurities and improving process yields, which is essential for consistent and high-quality manufacturing of end-use chemicals.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Speciality Chemical' category
 |
SHILPA CHEMSPEC INTERNATIONAL PRIVATE LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |