x

Malononitrile
1 INR/Kilograms
Product Details:
- Shape Crystalline Powder
- Physical Form Crystalline solid
- Smell Faint, characteristic odor
- Purity 99% min
- Melting Point 31-33C
- Density 1.12 Gram per cubic centimeter(g/cm3)
- Storage Keep tightly closed, store in cool and dry place, protect from moisture
- Click to View more
X
Malononitrile Price And Quantity
- 1 Kilograms
- 1 INR/Kilograms
Malononitrile Product Specifications
- Pharmaceuticals, Agrochemicals, Intermediate for organic synthesis
- Keep tightly closed, store in cool and dry place, protect from moisture
- 1.12 Gram per cubic centimeter(g/cm3)
- 109-77-3
- 2 years under proper storage conditions
- Slightly bitter
- Crystalline solid
- Crystalline Powder
- 99% min
- Faint, characteristic odor
- 31-33C
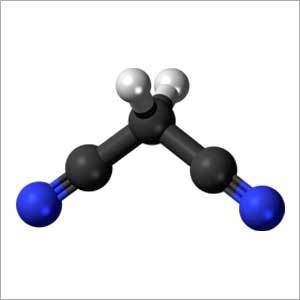
- NC-CH2-CN
- C3H2N2
- Highly polar, Volatile, Hygroscopic, Nitrile odor
- Neutral to slightly acidic
- White to slightly yellow crystals
- Soluble in water, ethanol, ether
- Industrial / Laboratory
- Malononitrile
- YES
- n20/D 1.441
- 66.06 g/mol
- 203-682-6
- Used in synthesis of pharmaceuticals, dyestuffs, amino acids, and other fine chemicals
- 29269000
- Organic intermediate / Nitrile compound
Malononitrile Trade Information
- 25 Kilograms Per Week
- 3-4 Week
- All India
Product Description
Malononitrile
| Test | Result | Unit | Specification |
|---|---|---|---|
| Assy(calculated;GC,KF) | 99.7 | % | >=99.0 |
| Water (KF) | <0.02 | %W/W | <=0.10 |
| 3-CPN(GC) | <0.02 | %area | <=0.10 |
| LZ-2871(GC) | 0.02 | %area | <=0.50 |
| MADN(GC) | 20 | %area | <=0.50 |
| BDN(GC) | 0.02 | %area | <=0.50 |
| Impurit..sum of oth. before mainpk.(GC) | 0.05 | %area | <=0.50 |
| Impurit..sum of oth. after mainpk.(GC) | <0.02 | %area | <0.50 |
Key Properties and Physical Characteristics
Malononitrile features a neutral to slightly acidic pH, a melting point of 31-33C, and a boiling point of 220C (with decomposition). Its density is 1.12 g/cm and it is highly soluble in water, ethanol, and ether. The compound has a refractive rate of n20/D 1.441 and emits a faint, nitrile odor. These properties make it versatile for various industrial and laboratory applications.
Storage and Stability Guidelines
For optimal stability, malononitrile should be tightly sealed and stored in a cool, dry environment, away from moisture and strong oxidizers. Proper storage not only preserves its purity but also ensures a shelf life of up to two years. Its packaging options include HDPE drum, fiber drum, or bag packing, tailored to industrial requirements.
Safety Precautions and Handling
Malononitrile is toxic if swallowed, inhaled, or in contact with skin, and carries the signal word Danger. It is regulated as a hazardous chemical, with UN Number 2811 and EC Index Number 608-005-00-4. Use protective equipment and handle only in well-ventilated areas to minimize exposure. Emergency procedures and compatibility assessments are essential during handling.
Applications in Industry and Research
Widely utilized as an intermediate in organic synthesis, malononitrile plays a vital role in manufacturing pharmaceuticals, agrochemicals, amino acids, and dyestuffs. Its highly polar nature and reactivity make it suitable for diverse chemical processes, benefiting research labs and industrial manufacturing alike.
FAQs of Malononitrile:
Q: How should malononitrile be safely stored to maintain its stability and shelf life?
A: Malononitrile must be kept tightly sealed in a cool, dry place, protected from moisture and incompatible substances such as strong oxidizers. Proper storage using suitable containers like HDPE or fiber drums ensures stability and preserves its shelf life for up to two years.Q: What are the main uses and industrial applications of malononitrile?
A: Malononitrile serves as an intermediate in the synthesis of pharmaceuticals, agrochemicals, amino acids, and dyestuffs. Its highly polar and reactive nature makes it beneficial for developing various fine chemicals in both industrial and laboratory settings.Q: When is malononitrile considered hazardous during handling or transport?
A: Malononitrile is classified as hazardous due to its toxicity by ingestion, inhalation, or skin contact. It is regulated under UN Number 2811 and must be transported according to hazardous chemical regulations, using appropriate packaging and safety measures.Q: Where can malononitrile be sourced and in what packaging forms is it available?
A: Malononitrile is distributed, imported, and supplied in India by manufacturers, suppliers, traders, and importers. It is available in HDPE drum, fiber drum, or bag packing, catering to both industrial and laboratory needs.Q: What precautions should be taken during the process and usage of malononitrile?
A: Personal protective equipment (PPE) should be worn, and work areas must be well-ventilated. Avoid contact with skin or eyes and do not inhale dust. Ensure compatibility by keeping it away from strong oxidizers, and have emergency response procedures accessible.Q: What benefits does malononitrile offer for organic synthesis and research?
A: Malononitriles high purity, polarity, and solubility make it an excellent reagent and intermediate for a broad range of chemical reactions. These attributes enhance accuracy and efficiency in research and industrial chemical synthesis.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Speciality Chemical' category
 |
SHILPA CHEMSPEC INTERNATIONAL PRIVATE LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |