x

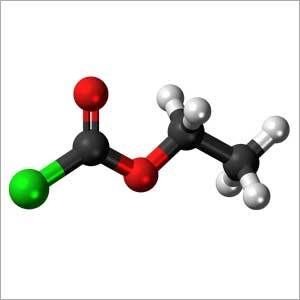
Ethyl Chloroformate
1 INR/Kilograms
Product Details:
- Properties Colorless liquid, highly reactive, efficient reagent for introduction of ethoxycarbonyl group, releases toxic gases on decomposition.
- Product Type Organic Chemical
- Storage Store in cool, dry, well-ventilated area, away from moisture
- Refractive Rate 1.399
- Density 1.105 Gram per cubic centimeter(g/cm3)
- Shape Liquid
- Melting Point -2 C
- Click to View more
X
Ethyl Chloroformate Price And Quantity
- 1 Kilograms
- 1 INR/Kilograms
Ethyl Chloroformate Product Specifications
- Pungent, characteristic odor
- Used in synthesis of pharmaceuticals, pesticides, and dyes
- Reagent for introduction of ethoxycarbonyl group
- Liquid
- 1.105 Gram per cubic centimeter(g/cm3)
- C3H5ClO2
- -2 C
- Store in cool, dry, well-ventilated area, away from moisture
- Organic Chemical
- 1.399
- Colorless liquid, highly reactive, efficient reagent for introduction of ethoxycarbonyl group, releases toxic gases on decomposition.
- 12 months
- Liquid
- 99%
- 208-791-3
- Technical/Analytical Grade
- Decomposes in water, soluble in alcohol and ether
- C2H5OCOCl
- 29159090
- 541-41-3
- Clear colorless liquid
- Ethyl Chloroformate
- 108.53 g/mol
- YES
Ethyl Chloroformate Trade Information
- 25 Kilograms Per Week
- 3-4 Week
- All India
Product Description
Ethyl Chloroformate (ECF)
Ethyl Chloroformate (MCF) finds appplication in Bulk Drug & Chemicals Intermediates manufacturing.
| Analysis Item | Standard | Result |
|---|---|---|
| Content | 99% | 99.50% |
| Carbpnate | 0.50% | 0.20% |
| Free Cl2 | 0.30% | 0.09% |
| Ethanol | 0.50% | 0.05% |
| Phosgene | 0.10% | 0.05% |
| Appearance | Colorless To Light Yellow Transparent Liquid | Colorless To Light Yellow Transparent Liquid |
| Color(hazen) | 50 | 30 |
| Conclusion | Conform | Conform |
Versatile Reagent for Chemical Synthesis
Ethyl Chloroformate is favored in organic synthesis for its ability to efficiently introduce ethoxycarbonyl groups. Its high purity (99%) and reactive nature make it ideal for pharmaceutical, pesticide, and dye manufacturing. Technically graded for industrial and analytical use, it offers reliable outcomes within specialized laboratory and production environments.
Safe Handling and Storage Recommendations
Due to its corrosive and harmful properties, Ethyl Chloroformate must be handled using proper protective equipment and stored in tightly sealed containers. Ensure storage in cool, dry, well-ventilated areas, strictly away from moisture, strong bases, and oxidizing agents to maintain its stability and prolong shelf life, which is typically 12 months.
Transport and Regulatory Compliance
Ethyl Chloroformate is classified under UN No. 1185, Class 6.1 (toxic), Packing Group II. Only authorized distributors, importers, and handlers should oversee its transport. Compliance with hazard statements and relevant safety protocols is mandatory during shipping and transit, particularly within India and for international transfer.
FAQs of Ethyl Chloroformate:
Q: How should Ethyl Chloroformate be safely stored to maximize its shelf life?
A: Store Ethyl Chloroformate in well-sealed glass bottles or drums, kept in cool, dry, and well-ventilated locations, away from moisture, strong bases, and oxidizers. Proper storage conditions help retain its stability and ensure a shelf life of up to 12 months.Q: What are the primary industrial applications of Ethyl Chloroformate?
A: Ethyl Chloroformate is extensively used as a reagent in the synthesis of pharmaceuticals, pesticides, and dyes, primarily for introducing the ethoxycarbonyl group into organic compounds due to its high reactivity.Q: When does Ethyl Chloroformate decompose, and what precautions should be taken?
A: This compound rapidly decomposes on exposure to moisture and releases toxic gases. Handle it in dry conditions using protective equipment, and ensure all containers are tightly sealed to mitigate risks associated with decomposition.Q: Where should Ethyl Chloroformate be kept within a facility to ensure safety?
A: Ethyl Chloroformate should be stored in a designated chemical storage area, away from incompatible substances and sources of moisture, ideally in an environment with controlled temperature and ventilation for maximum safety.Q: What is the process for handling Ethyl Chloroformate during transport?
A: Transportation requires compliance with UN No. 1185 regulations, Class 6.1 (toxic), Packing Group II. Only authorized personnel should handle the chemical, using certified containers and following all legal hazard communication protocols.Q: How is Ethyl Chloroformate typically used in chemical synthesis?
A: It serves as a potent reagent for introducing ethoxycarbonyl groups into organic molecules, facilitating crucial steps in the production of pharmaceutical ingredients, dyes, and various agrochemicals.Q: What are the benefits of using Ethyl Chloroformate in industrial processes?
A: Ethyl Chloroformate offers high efficiency and purity in chemical transformations, especially where ethoxycarbonylation is required, making it valuable in pharmaceutical, dye, and pesticide synthesis due to its reactive nature and reliable performance.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Speciality Chemical' category
 |
SHILPA CHEMSPEC INTERNATIONAL PRIVATE LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |