x

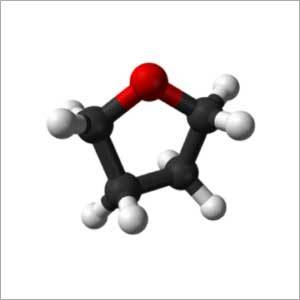
Tetrahydrofuran
1 INR/Kilograms
Product Details:
- Refractive Rate 1.407 (at 20C)
- EINECS No 203-726-8
- Molecular Formula C4H8O
- Shelf Life 2 years
- Storage Store in a cool, well-ventilated place, away from heat, sparks, open flames, and sources of ignition. Keep container tightly closed.
- CAS No 109-99-9
- HS Code 29321100
- Click to View more
X
Tetrahydrofuran Price And Quantity
- 1 INR/Kilograms
- 1 Kilograms
Tetrahydrofuran Product Specifications
- Neutral
- 99.9%
- -108 C
- Used as a solvent for PVC, polyvinylidene chloride, and in pharmaceutical synthesis.
- Industrial Grade
- Liquid
- 109-99-9
- 29321100
- Miscible with water and most organic solvents
- Store in a cool, well-ventilated place, away from heat, sparks, open flames, and sources of ignition. Keep container tightly closed.
- No (but harmful by inhalation, ingestion, or skin absorption)
- O / \ / \ | | \ / \____/
- Solvent for organic synthesis, adhesives, coatings, resins, and in electrolytes for batteries.
- Liquid
- Highly flammable, colorless, water-miscible, low viscosity, good solubilizing power for many polymers.
- 72.11 g/mol
- Ether-like odor
- 0.888 Gram per cubic centimeter(g/cm3)
- Organic Solvent
- Clear
- Tetrahydrofuran (THF)
- 203-726-8
- 1.407 (at 20C)
- C4H8O
- 2 years
Tetrahydrofuran Trade Information
- 25 Kilograms Per Week
- 3-4 Week
- All India
Product Description
Tetrahydrofuran (THF)
| Analysis Item | Unit | Specification |
|---|---|---|
| Appearance | .. | Clear Liquid |
| Purity | Wt % | 99.50 |
| Color | APHA | <5 |
| Moisture | Ppm | 94 (<0.01 by Wt%) |
| Peroxides | Ppm | 15 |
| Specific Gravity | 20/4 oC | 0.88745 |
| Refractive Index | ND20 oC | 1.4072 |
| 2, 3 Dihydrofuran | % | 0.0401 |
| 3 Methyl THF | % | 0.0071 |
Applications of Tetrahydrofuran (THF)
- Product is Stabilized with 200-350 ppm BHT.
- Tetrahydrofuran distilled pure has many applications as a solvent, reaction medium or starting product for syntheses. Only a few of the many examples of its usefulness are presented below.
- Coatings Artificial leather: PVC or polyurethane spread-coating systems contain THF alone or in blends with other solvents. Magnetic tapes: The abrasion resistance of video, computer or audio tapes can be improved by applying coatings derived from polyurethane or PVC in which the solvent phase is a blend of THF and toluene.
- Cellophane: The mechanical strength, impermeability to water vapor, heatsealability and printability of cellophane can be improved by applying coatings formulated from poly(vinylidene chloride) (PVDC), in which the solvent phase is a blend of THF and toluene.
- Adhesives: Tetrahydrofuran is suitable for the production of PVC adhesives for rigid PVC. It is used as a swelling agent for bonding plasticized PVC film and for fabricating PVC pipe systems. In both cases, the evaporation rate can be regulated by blending the THF with another solvent, such as cyclohexanone. Specialty Coating Systems By virtue of its excellent solvent power, THF allows the preparation of highly concentrated solutions of various polymers. These solutions can be diluted to the appropriate final concentration by adding thinners. Although THF evaporates rapidly, films formed from its solutions have very minimal tendency toward blushing during drying.
- Reaction Medium Tetrahydrofuran is a reaction medium, used primarily by the pharmaceutical industry, e. g., in Grignard syntheses or lithium aluminum hydride reductions. In fact, it is the only medium in which many Grignard compounds can be obtained. By Virtue of its good solvent power for alkali metals, THF is a useful reaction medium for the production of organometallic compounds.
- Anionic polymerization reactions can be carried out in THF as well. Extractant Some drugs, e. g., alkaloids, can be obtained in the pure form from their natural or synthetic precursors by extraction with THF.
- Also, impurities such as fat, wax or substances with a high molecular mass can be removed from compounds that are insoluble in THF.
- Starting Material for Syntheses Tetrahydrofuran is the starting material for a number of syntheses. For instance, ethers with a high molecular mass and chains of various lengths can be obtained by polymerization. The oxygen in the pentagonal ring can be replaced by nitrogen or sulfur to yield other five-membered heterocycles such as tetrahydrothiophene or pyrrolidine. The ring can be cleaved with halocarbons, e. g., 1,4-dihalobutane, or by oxidation (succinaldehyde and butyrolactone).
- Along with above applications THF also finds wide applications in Pharmaceutical industry.
Key Features and Physical Properties
THFs low viscosity (0.48 mPas), clear appearance, and ether-like odor make it easy to handle in various industrial and laboratory contexts. It is highly miscible with water and most organic solvents, enabling efficient dissolution and mixing. With a density of 0.888 g/cm and a melting point of -108 C, it remains stable under normal conditions when proper inhibitors are added.
Safety and Storage Guidelines
As a Class 3 flammable liquid (UN 2056), THF requires secure storage in tightly closed containers, away from ignition sources and sunlight. The addition of inhibitors such as BHT (0.025%) prevents the dangerous formation of peroxides. Regular ventilation and cool temperature are essential for safety. Shelf life extends up to two years under recommended conditions.
Applications and Benefits
THF is invaluable for dissolving polymers like PVC, facilitating pharmaceutical synthesis, and serving as a solvent in adhesives, coatings, resins, and battery electrolytes. Its excellent solubilizing power and compatibility with other solvents enhance versatility, making THF a preferred choice across industries ranging from manufacturing to laboratory research.
FAQs of Tetrahydrofuran:
Q: How should Tetrahydrofuran be stored to ensure safety and product integrity?
A: Tetrahydrofuran should be stored in a cool, well-ventilated area, in tightly closed metal or HDPE drums, and away from heat, sparks, open flames, and oxidizers. Inhibitors such as BHT are added to suppress peroxide formation. Follow local regulations for flammable substances during storage.Q: What are the main applications of Tetrahydrofuran in industry and research?
A: THF is primarily used as a solvent for PVC, polyvinylidene chloride, and in pharmaceutical synthesis. It is also employed in manufacturing adhesives, coatings, resins, and as an electrolyte in batteries, thanks to its exceptional solubilizing properties.Q: When is Tetrahydrofuran considered hazardous, and what risk mitigation steps are recommended?
A: THF is highly flammable and can form explosive peroxides upon exposure to air. Use protective equipment, ensure adequate ventilation, handle it away from ignition sources, and regularly monitor inhibitor levels to reduce peroxide risk. Proper storage and safety measures are vital.Q: Where can Tetrahydrofuran be transported or shipped, and what are the regulatory considerations?
A: THF can be transported internationally by road, rail, sea, or air under UN Number 2056 guidelines. Adherence to ADR/RID/IMDG/IATA regulations is mandatory, and packaging must comply with local and international standards for flammable liquids.Q: What is the benefit of using Tetrahydrofuran as a solvent compared to alternatives?
A: Tetrahydrofuran offers superior miscibility with water and organic solvents, low viscosity, and an ability to dissolve a wide range of polymers, making it an efficient and versatile solvent for various manufacturing and research purposes.Q: Is Tetrahydrofuran environmentally hazardous, and how should waste be managed?
A: THF is not classed as environmentally hazardous but should not be released into the environment without proper permits. Dispose of THF waste according to local environmental regulations, and avoid direct discharge to prevent potential harm.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Speciality Chemical' category
 |
SHILPA CHEMSPEC INTERNATIONAL PRIVATE LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |